Abstract
Introduction
Evading apoptosis is one of the key mechanisms that cells become cancerous. Oncogenic proteins in the family, including BCL-2, BCL-xL, and MCL-1, which counteract the pro-death function of tumor suppressor like proteins, have been shown in many models of tumorigenesis. Thus how to reprogram the apoptosis regulatory machinery to tilt towards pro-death activity has been the focus of modern cancer therapy, regardless of conventional or targeted therapy. Inhibiting BCL-2, BCL-xL and/or MCL-1 anti-death proteins has also become the hot pursuit for drug development. To effectively kill cancers with heterogeneity, it is necessary to understand which oncogene is the underlying mechanism. Applying novel BCL-2 inhibitors, a selective or dual inhibitor for BCL-2/BCL-xL, which have been developed by Ascentage Pharma Group and currently under phase I/II clinical trials, we probed the oncogene addiction patterns in a panel of hematologic malignancy cell lines, including ALL, AML and MM.
Methods
Cell sensitivity to BCL-2 inhibitors was assessed by Cell-titer-glow assays. 2. Protein expression levels by Western blotting, or protein complexing detected by MSD method were used to dissect the mechanism of sensitivity or resistance. 3. Mice xenograft models were carried out to confirm the tumor growth inhibition (efficacy model) and regression (survival model) through combination treatments. 4. RNAseq was performed to reveal the transcriptome alteration by APG-115, a novel MDM2 inhibitor also developed by Ascentage, to re-sensitize cells to BCL-2 inhibitors.
Results
A panel of hematologic malignancy cell lines were first assessed cells' sensitivity to novel BCL-2/ BCL-xL inhibitors, and revealed a distinct pattern of dependence on BCL-2, BCL-xL or MCL-1, from the most sensitive ALL line RS4;11 to BCL-2, to the most resistant one like MM line H929. In between there is a mix pattern of dependence on these three anti-death proteins.
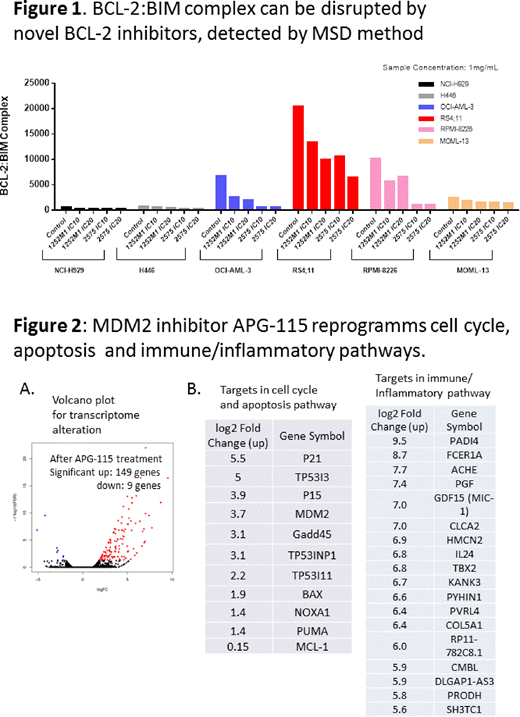
To dissect the mechanism of action, we established and validated BCL-2:BIM complex using MSD method. In BCL-2 dependent cell lines, BCL-2:BIM complex correlates with cells sensitivity to BCL-2 selective inhibitor. While in the mix pattern, high BCL-2:BIM complex alone does not predict drug sensitivity. Pretreating cells with BCL-2 inhibitors, either selective or dual inhibitors, can disrupt the BCL-2:BIM complex and reduce signals detected by MSD methods (Figure 1). Such reduction is consistent with co-immunoprecipitation results using Ascentage selective BCL-2 inhibitor or reference compound ABT-199, suggesting BCL-2 protecting cells through sequestering pro-death protein BIM.
To overcome resistance to BCL-2 inhibitor, we combined it with our novel MDM2 inhibitor APG-115. Both in vitro cell viability assay and mice xenograft models demonstrated that MDM2 and BCL-2 inhibitor combination can re-sensitize cells and effectively inhibit tumor growth or lead to tumor regression.
To obtain a full transcriptome profile changed by APG-115, we further performed RNAseq on OCI-AML3, which is intrinsically resistant to BCL-2 inhibitor. RNAseq results revealed that APG-115 reprogramed cell cycle and apoptosis pathway, increasing its known targets for cell cycle regulation (p21, p15 protein) and pro-death proteins (BAX, PUMA and NOXA). Furthermore, APG-115 also increased significantly some target genes involved in immune or inflammatory response pathway, including MIC-1 (macrophage inhibitory cytokine-1, GDF15), IL-24 (also known as mda-7, melanoma differentiation-associated protein 7, tumor suppressing protein), and PADI4 (peptidyl arginine deiminase, type IV, regulating granulocyte and macrophage via histone remodeling), which may alter tumor microenvironment and manifest cell death signals (Figure 2).
Conclusions
Using novel selective or dual BCL-2/BCL-xL inhibitor, we probed the oncogene addiction patterns in a panel of hematologic malignancy cell lines, and revealed a mix pattern of dependence on BCL-2, BCL-xL or MCL-1. The BCL-2 addiction relies on the sequestration of pro-death proteins like BIM. Disrupting BCL-2:BIM complex by BCL-2 inhibitors can trigger cell death. MDM2 inhibitor APG-115 reprograms cell cycle, apoptosis and immune/inflammatory pathways to re-sensitize cells to BCL-2 inhibitor.
Deng:Ascentage Pharma Group: Employment. Yin:Ascentage Pharma Group: Employment. Mao:Ascentage Pharma Group: Employment. Zhai:Ascentage Pharma Group: Employment. Yang:Ascentage Pharma Group: Employment. Fang:Ascentage Pharma Group: Employment. Wang:Ascentage Pharma Group: Employment. Zhai:Ascentage Pharma Group Inc.: Employment. Yang:Ascentage Pharma Group Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal